- Joined
- Dec 20, 2009
- Messages
- 4,669
- Reaction score
- 6,790
Registered Members experience this forum ad and noise-free.
CLICK HERE to Register for a free account and login for a smoother ad-free experience. It's easy, and only takes a few moments.It kill me that most of the talking heads are not really addressing the science heads on. If the ball deflation is explained by the temperature drop, then the texts, phone calls, etc all become irrelevant. So, I've tried to simplify how the perfect gas law applies to this.
Perfect gas law says that absolute ball pressure is directly proportional to absolute temperature assuming ball volume is constant. If absolute temperature were to increase 50%, absolute ball pressure would go up by 50%.
Absolute ball pressure is measured ball pressure plus ambient atmospheric pressure.
Absolute temperature is measured temperature converted to Degrees Kelvin.
Locker room temperature of 72 Deg F converts to 295 Deg K
Playing field temperature of 45 Deg F converts to 280 Deg K, a 5% drop.
Now, given that ball pressure is directly proportional to temperature, let’s apply the 5% drop to the pressure.
Ambient air pressure at sea level is 14.7 psi.
Absolute ball pressure measured in 72 Deg F locker room is 12.5 psi + 14.7 psi = 27.2 psi
Apply 5% drop to that and you get 25.8 psi at 45 Deg F.
Subtract the ambient air pressure (14.7) and you get 11.1 psi.
NOT THAT COMPLICATED!
Now, if you consider the ball got wet, it gets a bit more complicated. The wet ball would soften the leather, allowing it to expand a bit, thus increasing its volume, resulting in a somewhat even lower air pressure in game conditions.
Reference:
PV=nRT
P=Absolute Pressure (air gauge pressure plus ambient pressure)
V=Volume (volume of a dry football is constant across range of temperature)
n=number of molecules of air in the football (constant across the range of temperature)
R=Gas Constant. It's a constant, like pi.
T=Temperature in Deg K.
P=(nr/V)T
nr/V = K (constant)
P=KT
P directly proportional to T
EDIT 8/4/15
I've been called out because the temperature values I used were not the ones reported in the Wells Report. That's a valid observation; the number I chose were to illustrate a point. So I recalculated using the Wells Report data: Locker Room Temp 71-74, Field Temp 48-50. I calculated the four permutations using the min and max for each reported range with the starting ball pressure set to 12.5 psi.
Locker Room-Field-Ball Pressure
71-----------48-----------11.32
74-----------50-----------11.28
71-----------50----------- 11.42
74---------- 48------------11.17
Calculated Average------11.30
Wells Report Average---11.49 (Logo), 11.11 (Non Logo)
I was called out for not using the Wells temperature numbers, so I've updated the original post to reflect what was in the report. The original point stands: observed ball deflation is consistent with the Natural Gas Law.
There are so many unsupported assumptions in the Wells report: the temperatures were not recorded, the initial ball pressures were not recorded, the locker room temperature was not recorded, which gauge was used was not recorded. It all makes for bogus science, recognized even by the NFL given their new ball handling procedures released just recently.so you were called out by not using the temperature number that was MADE UP in order to produce the result?
How bizarre is that.
Shouldn't the investigation be what unknowns would have to happen for their to be no tampering rather than what can we assume happened in order to 'get a guilty'?
With children under the age of 13, if they decided to get rid of common core math my household would be a helluva lot calmer....Goodell was probably using the common core to do the math, that's why he got it wrong.
The 71-74 degree locker room temperature??? Did the Wells Report specify how these temperature numbers were determined? Cold wet day....refs returning to a low 70's locker room? If I'm cold and drenched and likely to change wardrobe for the 2nd half, I would have cranked that locker room temperature up.8) The 4 Colts footballs tested indeed dropped less in pressure, simply because of when they were tested, at the very end of halftime, after they sat in a warm room for probably at least 12 minutes.
There are so many unsupported assumptions in the Wells report: the temperatures were not recorded, the initial ball pressures were not recorded, the locker room temperature was not recorded, which gauge was used was not recorded. It all makes for bogus science, recognized even by the NFL given their new ball handling procedures released just recently.
My point, though, is that even the bogus Wells numbers are consistent with the Natural Gas Law.
You are missing one big one: they don't know when they measured the Colts balls for certain. If the balls were measured a few minutes later then the Wells Team suggested they were then the results on the logo gauge also make sense for the Colts balls.
You are missing one big one: they don't know when they measured the Colts balls for certain. If the balls were measured a few minutes later then the Wells Team suggested they were then the results on the logo gauge also make sense for the Colts balls.
simplest possible thing to do is ask"explain this"
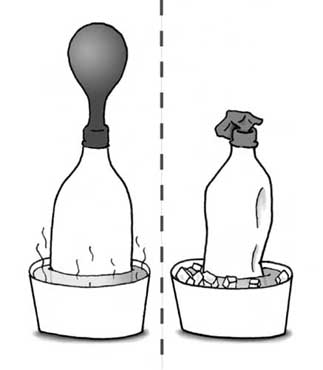
Your balloon has a hole in it?simplest possible thing to do is ask"explain this"

First of all, thanks for all that work.It kill me that most of the talking heads are not really addressing the science heads on. If the ball deflation is explained by the temperature drop, then the texts, phone calls, etc all become irrelevant. So, I've tried to simplify how the perfect gas law applies to this.
Perfect gas law says that absolute ball pressure is directly proportional to absolute temperature assuming ball volume is constant. If absolute temperature were to increase 50%, absolute ball pressure would go up by 50%.
Absolute ball pressure is measured ball pressure plus ambient atmospheric pressure.
Absolute temperature is measured temperature converted to Degrees Kelvin.
Locker room temperature of 72 Deg F converts to 295 Deg K
Playing field temperature of 45 Deg F converts to 280 Deg K, a 5% drop.
Now, given that ball pressure is directly proportional to temperature, let’s apply the 5% drop to the pressure.
Ambient air pressure at sea level is 14.7 psi.
Absolute ball pressure measured in 72 Deg F locker room is 12.5 psi + 14.7 psi = 27.2 psi
Apply 5% drop to that and you get 25.8 psi at 45 Deg F.
Subtract the ambient air pressure (14.7) and you get 11.1 psi.
NOT THAT COMPLICATED!
Now, if you consider the ball got wet, it gets a bit more complicated. The wet ball would soften the leather, allowing it to expand a bit, thus increasing its volume, resulting in a somewhat even lower air pressure in game conditions.
Reference:
PV=nRT
P=Absolute Pressure (air gauge pressure plus ambient pressure)
V=Volume (volume of a dry football is constant across range of temperature)
n=number of molecules of air in the football (constant across the range of temperature)
R=Gas Constant. It's a constant, like pi.
T=Temperature in Deg K.
P=(nr/V)T
nr/V = K (constant)
P=KT
P directly proportional to T
EDIT 8/4/15
I've been called out because the temperature values I used above were not the ones reported in the Wells Report. That's a valid observation; the number I chose were to illustrate a point. So I recalculated using the Wells Report data: Locker Room Temp 71-74, Field Temp 48-50. I calculated the four permutations using the min and max for each reported range with the starting ball pressure set to 12.5 psi.
Locker Room-Field-Ball Pressure
71-----------48-----------11.32
74-----------50-----------11.28
71-----------50----------- 11.42
74---------- 48------------11.17
Calculated Average------11.30
Wells Report Average---11.49 (Logo), 11.11 (Non Logo)
Note that the Wells Report is based on several unsupported assumptions: the field temperature was not recorded, the initial ball pressures were not recorded, the locker room temperature was not recorded, which gauge was used was not recorded. It all makes for bogus science, recognized even by the NFL given their new ball handling procedures released just recently.
My point, though, is that even the bogus Wells numbers are consistent with the Natural Gas Law.